Differential scanning calorimetry (DSC) is a thermal analysis technique in which the difference between the rate of heat flow into a specimen and that into a reference is measured as a function of temperature or time while the specimen and reference are subjected to the same temperature control program.
As an example, when the specimen melts during the heating process, the DSC signal exhibits an endothermic peak. The characteristic temperature and enthalpy of melting can be determined from the peak.
There are two different DSC measurement methods:
Heat Flux DSCs
A technique in which the temperature of the sample unit, formed by a sample and reference material, is varied in a specified program, and the temperature difference between the sample and the reference material is measured as a function of temperature.
Power Compensation DSC
A technique in which the difference of thermal energy that is applied to the sample and the reference material per unit of time is measured as a function of the temperature to equalize their temperature, while temperature of the sample unit, formed by the sample and reference material, is varied in a specified program.
Since the most popular DSCs are Heat Flux DSCs, we will focus on how explaining how these work. The sample to be analyzed and a reference are placed in a DSC furnace and the temperature of the furnace is changed under controlled conditions. The temperature difference between the sample and the reference material is measured as a function of the applied temperature.
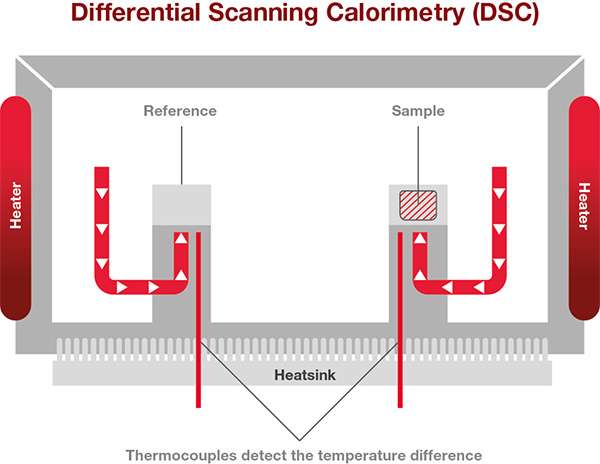
The difference in temperature between the sample and the thermally stable reference indicates changes of state within the sample.
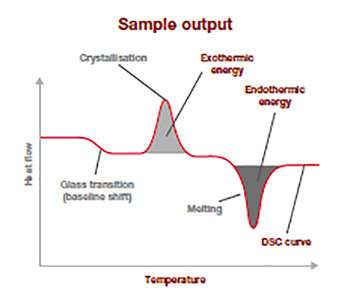
This sample output thermogram above illustrates the typical information from a DSC analysis run and is a plot of the difference in temperature (heat flow: y-axis(mW/mg)) in relation to the rising applied temperature (x-axis).
The baseline shift shows at which temperature the glass transition is happening and how much energy was required to make it happens. The position and spread of the exothermic peak give information on the cold-crystallization temperature and its energy released, and the endothermic peak relates to the temperature of fusion and how much energy was required to go through this thermal transitoon. This information allows to you to confirm for example polymer ID, quality, crystallinity and purity, and is an excellent raw material quality check.
A heat flux DSC comprises of the sample and reference holder, heat resistor, heat sink, and heater. Heat of the heater is supplied into the sample and the reference through heat sink and heat resistor. Heat flow is proportional to the heat difference of heat sink and holders. The heat sink has the enough heat capacity compared to the sample.
In case the sample goes through endothermic or exothermic phenomena such as transition and reaction. This endothermic or exothermic phenomena will temporarily make the sample temperature differ from the program temperature. Thus, the sample’s temperature will be different to the reference which is thermally inert. The difference in the sample and the reference temperature is proportional to energy required or released to go through a thermal event. By calibrating the instrument with a reference standard, the temperature and energy required to go through a thermal event can be measured accurately and precisely.
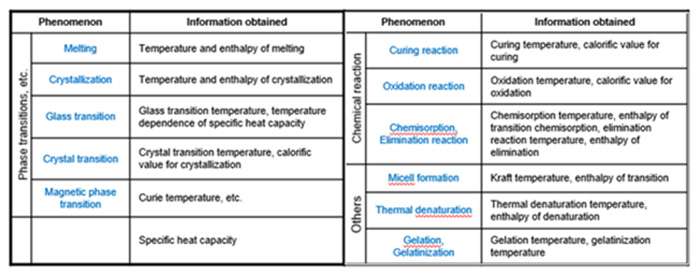
DSC enables the measurements transitions such as the glass transition, melting, and crystallization. Furthermore, the chemical reaction such as thermal & UV curing, heat history, specific heat capacity (Cp), and purity analysis are also measurable.
Recently, with the development of the highly functional polymeric material, the analysis of subtle changes in thermal properties have increased dramatically.
Here’s a list of phenomena that can be measured by DSC and the information obtained.
Two of the main fundamental material properties that determine the suitability of a material for a given application are glass transition and melting.
Glass transition (Tg) indicates the temperature at which a substance transforms from its glassy state (hard) to its rubbery state (soft) and vice versa. The glass transition will be present in materials containing an amorphous phase which lacks order. Its temperature and how much a material is amorphous can be controlled through its process or by adding additives which will give the required material mechanical property. Determining the glass transition provides the upper and lower temperature limits for materials for production parameter optimization, quality control and failure analysis.
Melting point (Tm) indicates the temperature points when the first detectable liquid phase is spotted to the temperature at which no solid material is left. Melting point can be detected in crystalline and semi-crystalline materials and its enthalpy of melting can be used to determine purity and crystallinity degree in materials.
We’ve picked ASTM E794-06(2018) standard test method for melting and crystallization temperatures by thermal analysis as our example to talk through interpreting the results.
This test method describes the determination of melting (and crystallization) temperatures of pure materials by differential scanning calorimetry (DSC) and differential thermal analysis (DTA).
The DSC thermograms bellow shows how this technique can differentiate between different type of polymers. Polymers have different melting temperatures depending on their type, but also to their composition (type or concentration of additives). The example below shows two types of polyethylene (high and low density) which exhibit different melting point temperature. For polymers, the melting temperature is taken at the peak of the melting process.
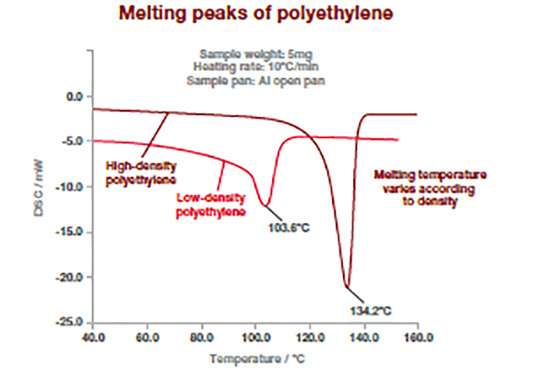
This measurement can be used to confirm incoming raw materials identification, detect trace of impurities (e.g., polypropylene in polyethylene) as well as final product specifications. It works for powders, films or pellets.
Probably the most popular of the four thermal analysis techniques, DSC’s wide temperature range enables detection of a broad range of transitions. It’s also easy to measure transitions in materials, especially for polymers for which glass transition is important.
Our NEXTA DSC instruments offer world-class sensitivity and baseline reproducibility, delivering more accurate testing and the ability to evaluate even the smallest thermal events. Our innovative Real View® camera system provides real-time observation during measurement which allows to colour analysis, dimension change measurement as well as understanding unexpected results. Having access to images of your sample during the measurement also helps to share results with your colleagues and customers who might not know this technique. The DSC200 offers leading technology for routine applications without limitations and ideal for a wide variety of applications. The DSC600 is purpose designed to meet the most advanced materials development and failure analysis thanks to its sensitivity and resolution, especially within applied research.
Watch our expert James Bennet give a demo of a DSC.
Discover moreYou might also be interested: