Polypropylene (PP) is an inexpensive crystalline polymer that is easy to mold into different shapes, is very strong and is chemical and water resistant. As such, it’s one of the most widely produced plastics and is used for packaging, as an additive to concrete structures, insulation of electrical cables and is the raw material for medical grade protective equipment, such as face masks.
 Find out more about our DSC instruments
Find out more about our DSC instruments Polypropylene components are molded by heating the plastic until it melts. The subsequent cooling affects the material crystallinity and must be controlled to ensure the right material properties such as brittleness are achieved. Differential scanning calorimetry (DSC) can be used to evaluate the temperature dependence of PP and the effects of the cooling profile and additives on the crystallinity of the material.
At Hitachi, we tested several samples of polypropylene to demonstrate how differential scanning calorimetry can be used to check how PP properties change under different processing conditions.
Experimental set up
We used commercially available polypropylene sheets as samples and used a Hitachi High-Tech NEXTA DSC to evaluate the thermal properties.
The first test was to evaluate the temperature dependence of the crystal structure. To do this, we evaluated four samples of the PP that had undergone four different thermal processes:
Sample 1: No treatment
Sample 2: Heating to 110ºC, then quenching
Sample 3: Heating to 115ºC, then quenching
Sample 4: Heating to 120ºC, then quenching.
Each sample in turn was then evaluated within the DSC instrument. They were heated from room temperature to 200ºC at 10ºC / min within a nitrogen atmosphere. Results from the DSC tests are presented in the following graphs:
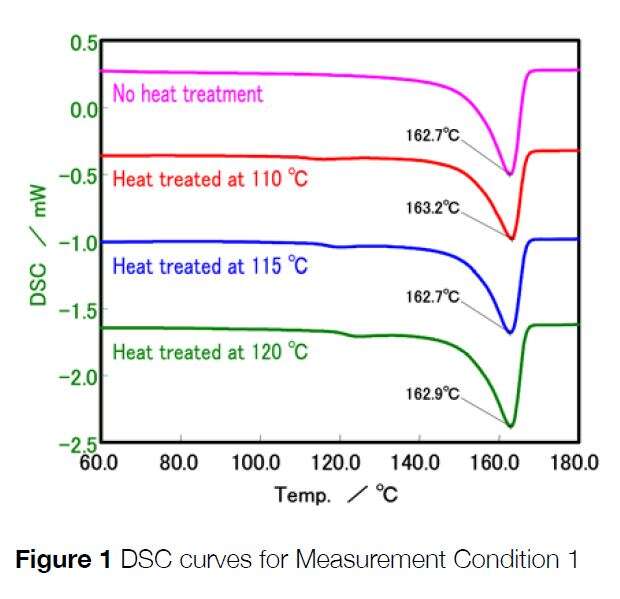
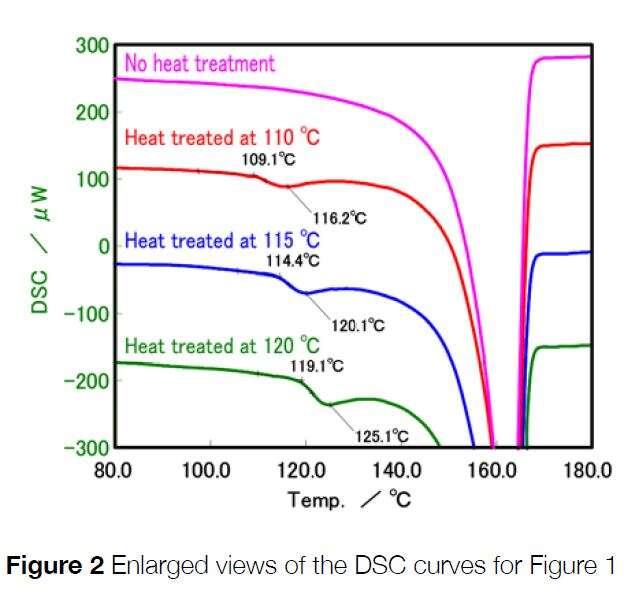
You can see from the results that all samples show an endothermic peak at around 160ºC which corresponds with the melting of the PP. If you look at the graph on the right that shows a zoomed in section, you can see differences between the four traces. The untreated sample is flat, whereas the heat-treated samples show very small endothermic peaks very close to their corresponding heat treatment temperature. This shows that each heat treatment produced a different crystal structure. By optimizing the injection molding line temperature, it is possible to control the final product mechanical properties as well as controlling the processing cost associated it.
Next, we’ll use the DSC to evaluate different cooling profiles on the crystallization time of the PP.
Identical samples of PP were taken and heated to 200ºC in a nitrogen atmosphere to melt them. They were then quenched to different temperatures and held at this temperature for 15 to 50 minutes. The graph below shows the output of DSC measurements for the different quenching temperatures.
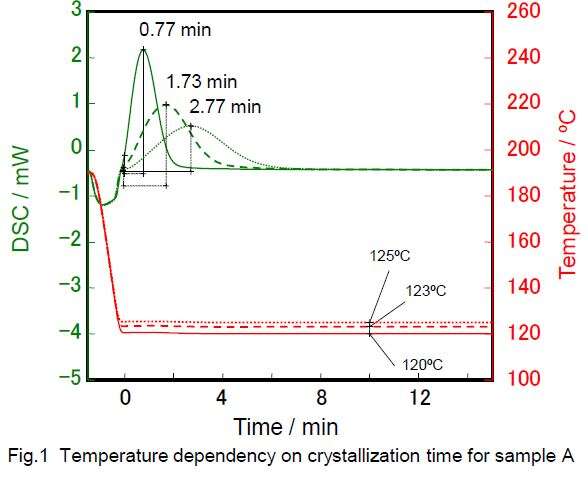
The graphs show that the PP crystallization produced an exothermic peak at each holding temperature. The lower the holding temperature, the sharper the peak and the sooner the crystallization occurred. Conversely, the higher the holding temperature during quenching, the broader the peak and more time elapsed before crystallization.
This is because the higher temperature makes crystallization more difficult, increasing the time for it to be completed. Since an increase in crystallization time will slow down production and could affect the final product properties (e.g. brittleness), it is important to optimize it.
Finally, we’ll show how DSC can be used to evaluate the effect of additives to the crystallization behavior.
Two different samples of PP, A and B, where sample B has an additive present. Both samples were heated to 200ºC and then quenched at a holding temperature of 125ºC. The graph below shows the DSC output:
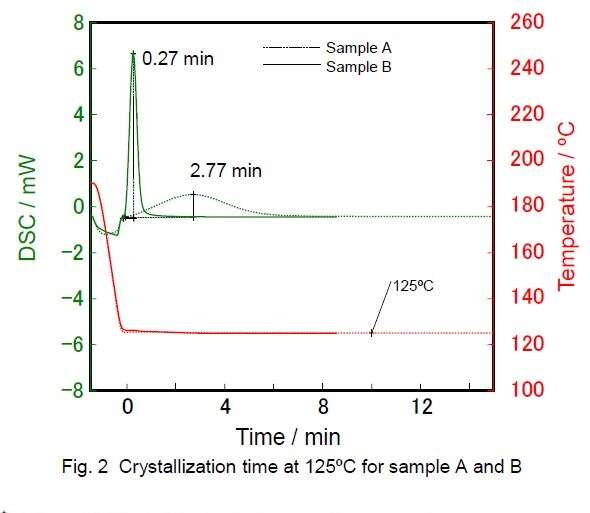
You can see a sharp difference in the time taken for crystallization to complete between the two samples. For the sample with the additive (B), crystallization is complete in under 2 minutes, whereas sample A took much longer. This method allows you to evaluate the effects of crystallization by additive used. Since additives are expensive, you want to make sure you are using the right one and with the exact amount that will give the required properties for your final products.
As we’ve demonstrated in these experiments, DSC is invaluable when optimizing processing time and temperature during PP molding.
The instrument used in this analysis was the NEXTA DSC, a high-sensitivity, versatile analyzer that’s used in a variety of applications, including polymer characterization. The NEXTA DSC range includes a unique furnace design that gives world-class baseline flatness and a RealView camera system that shows you material behavior live on screen.
To find out more about DSC for polymer material characterization, get in touch to arrange a demo.
Get in touch