X-rays form part of the electromagnetic spectrum. They are on the high energy side of ultraviolet, and are expressed in terms of their energy in kilo electron volts (keV), or wavelength in nanometers (nm). X-ray fluorescence (XRF) can typically analyse elements from sodium to uranium, in concentrations ranging from parts per million to high percents, in solids, liquids, and powders.
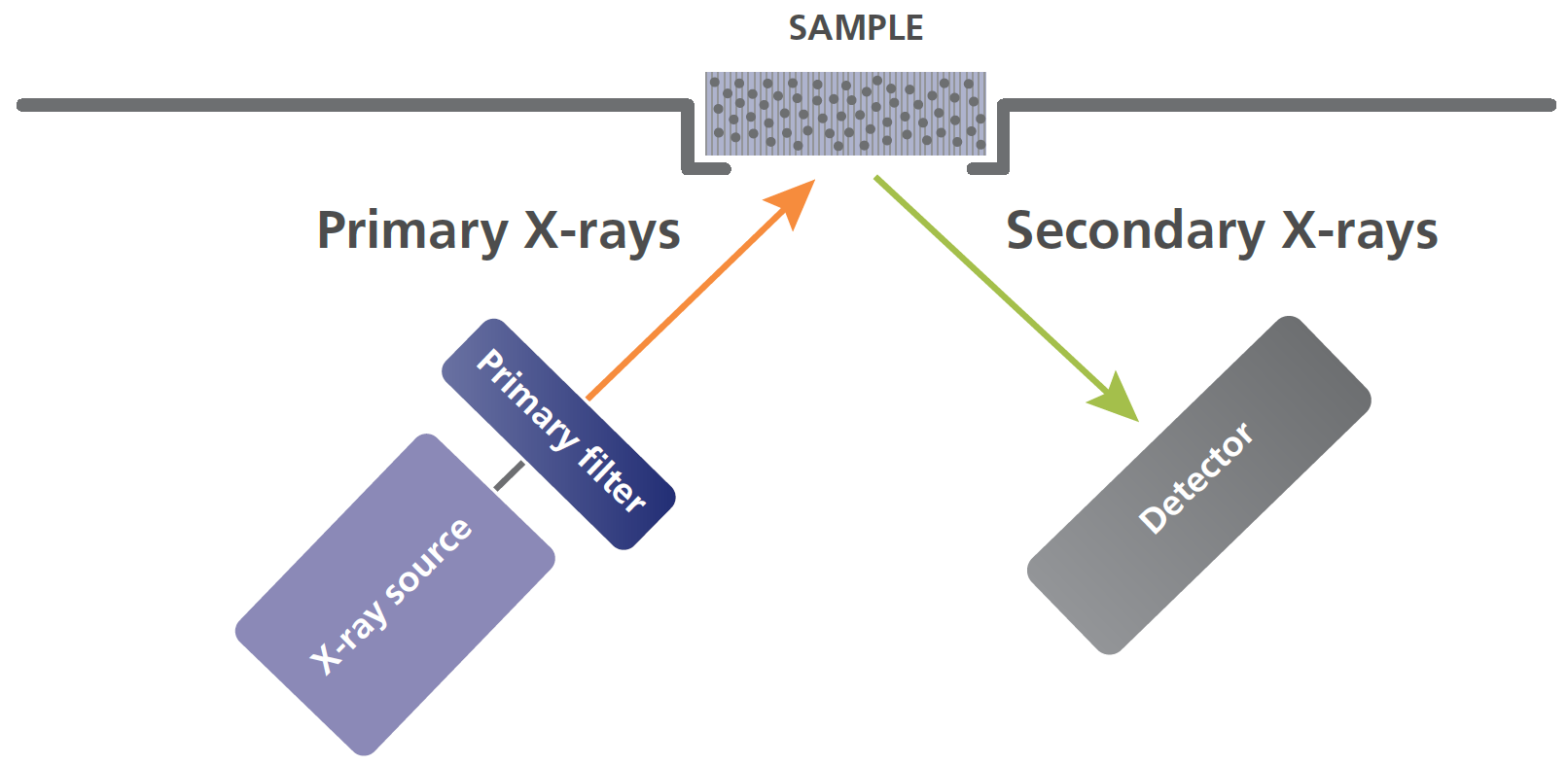
All XRF instruments are designed around two major components: an x-ray source (commonly an x-ray tube), and a detector.
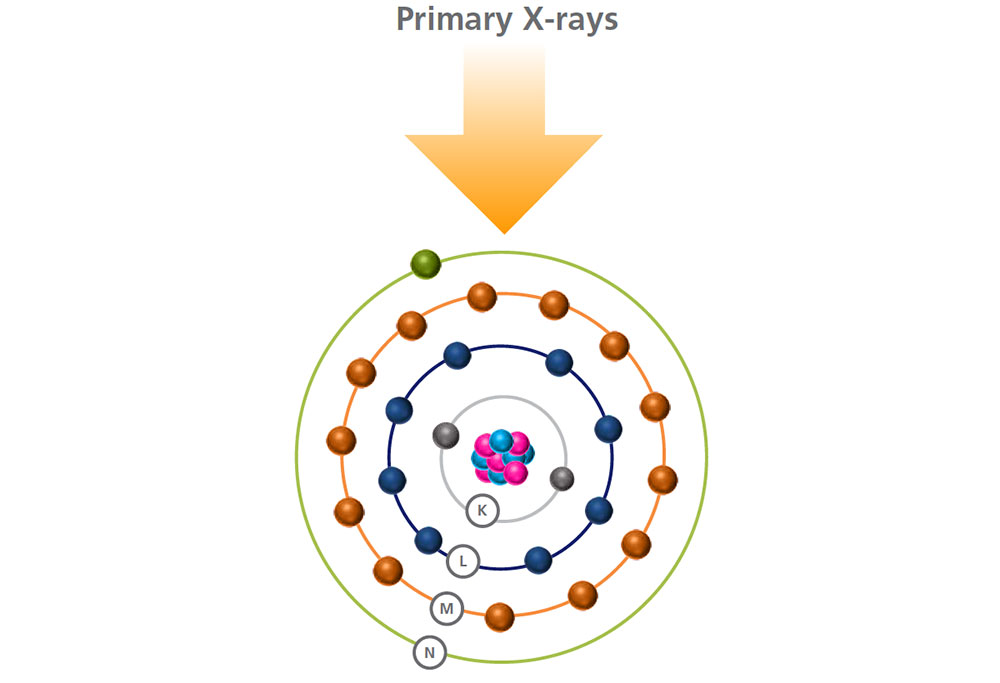
Primary x-rays are generated by the source and directed at the sample’s surface, sometimes passing through a filter to modify the x-ray beam.
When the beam hits the atoms in the sample, they react by generating secondary x-rays that are collected and processed by a detector.
The x-rays emitted by the atoms in the sample are collected by a detector, and processed in the analyser to generate a spectrum showing the x-rays intensity peaks versus their energy.
The peak energy identifies the element and its peak area (or intensity) gives an indication of its amount in the sample.
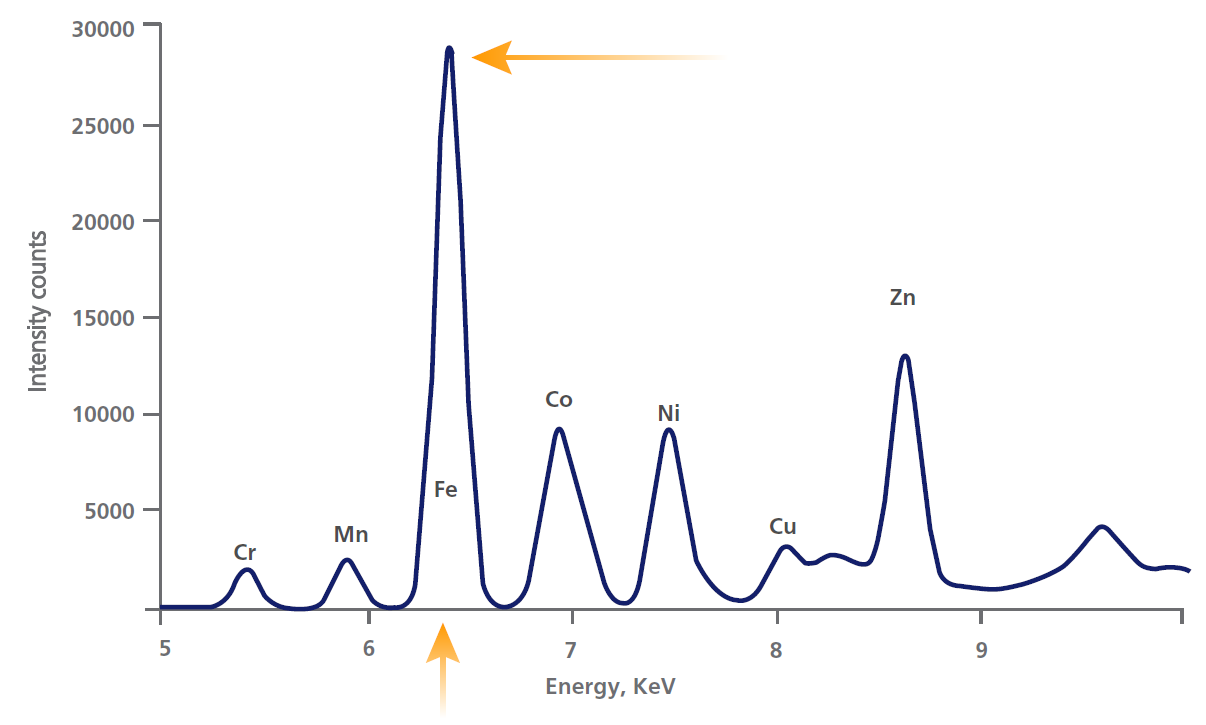
The analyser then uses this information to calculate the sample’s elemental composition.
The whole process, from pressing a start button or a trigger, to getting the analysis results, can be as quick as 2 seconds, or it can take several minutes.
It all depends on the instrument used, the range of elements measured, and the concentrations of those elements.
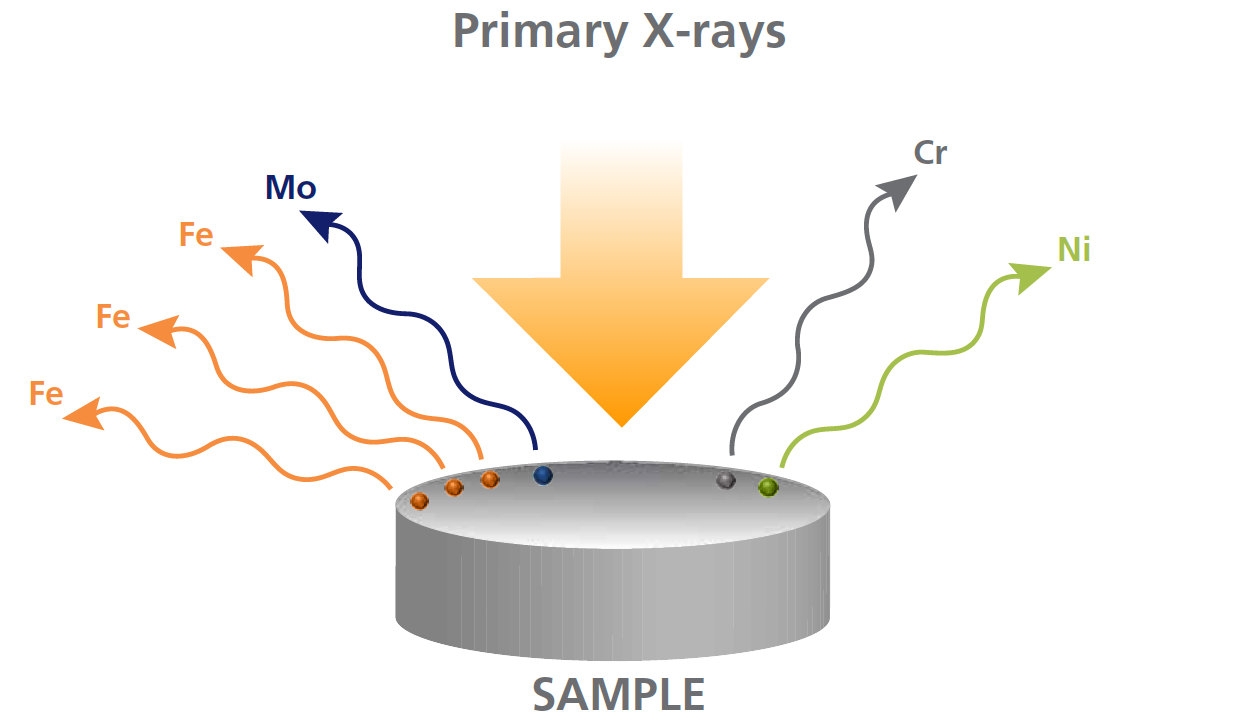
The energy of the emitted x-ray is characteristic of the element.
This means that XRF provides qualitative information about the sample measured.
However, XRF is also a quantitative technique.
What happens to the atoms in the sample during the analysis?
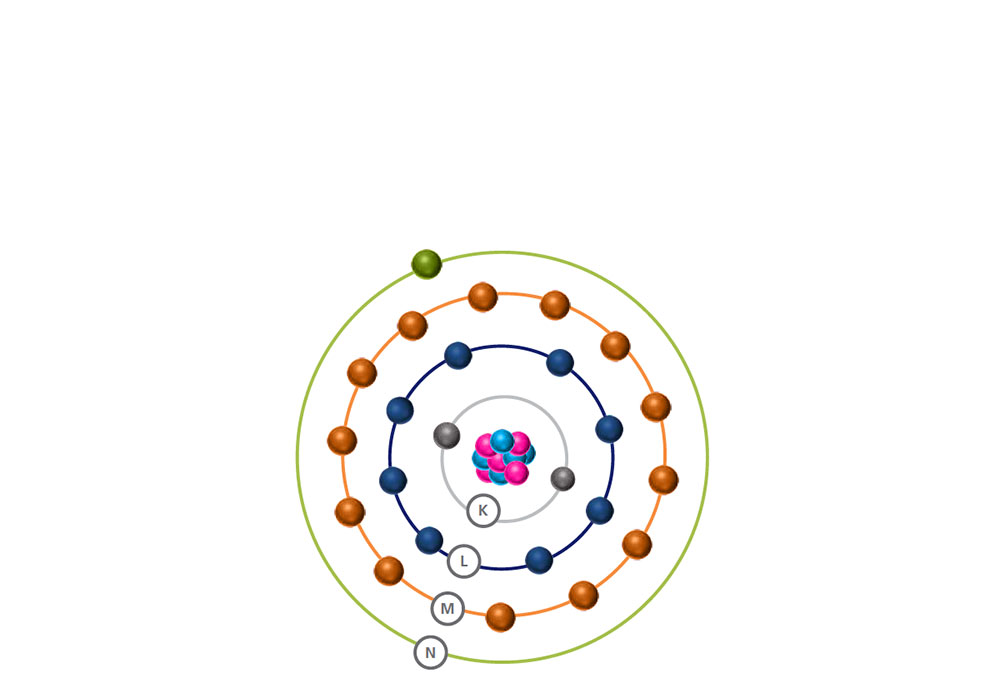
A stable atom is made of a nucleus and electrons orbiting it.
The electrons are arranged in energy levels or shells (K, L, M, N) and different energy levels can hold different numbers of electrons.
When a high energy primary x-ray collides with an atom, it disturbs its equilibrium.
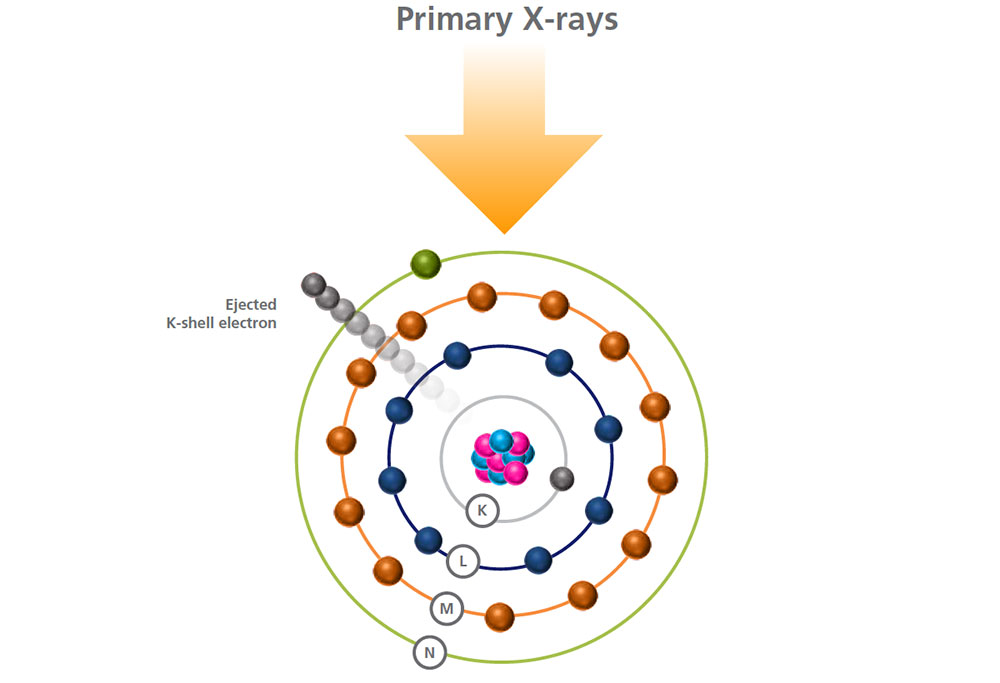
An electron is ejected from a low energy level, and a vacancy is created, making the atom unstable.
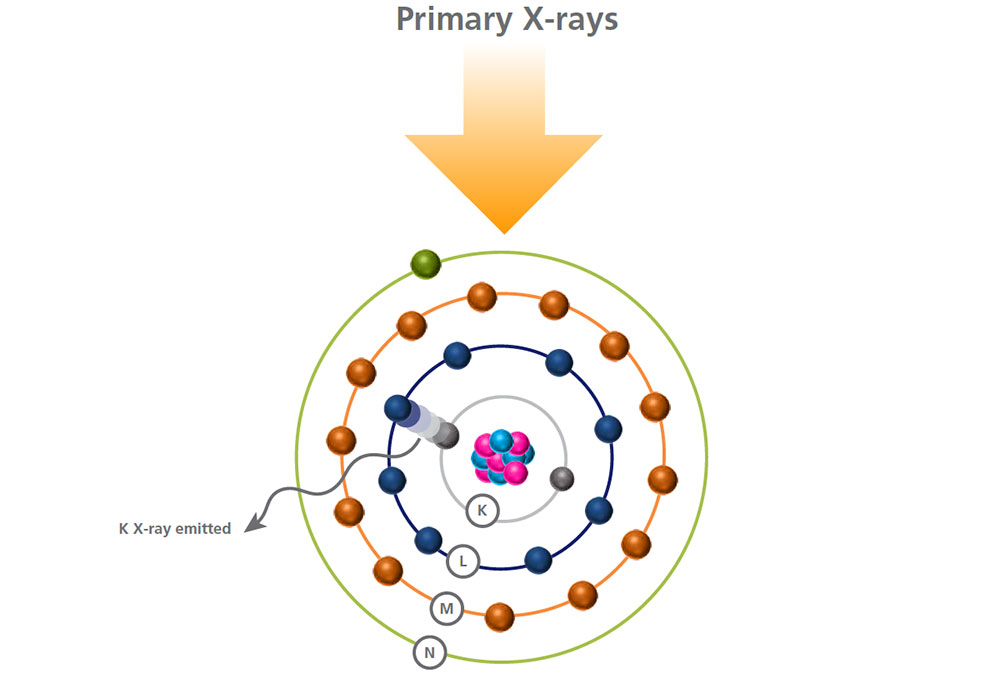
To restore stability, an electron from a higher energy level falls into this vacancy and the excess energy released as the electron moves between the two levels is emitted in the form of a secondary x-ray.